Projects
- Uncovering mechanisms of T-cell co-stimulation/inhibition. Co-receptors such as CD28, CTLA-4, ICOS and PD-1 play key roles in modifying signals from the antigen-receptor complex in various disease contexts. My laboratory has played a leading international role in uncovering the mechanisms by which these co-receptors signal to modify T-cell responses. We first defined CD28, ICOS and CTLA-4 binding to lipid kinase phosphatidylinositol 3-kinase (PI 3K) and the adaptor Grb2 which is now widely quoted in textbooks and is responsible for CD28 activation of the inflammatory NFκb pathway in T-cells. A major project in the lab is to uncover the signaling pathways linked to CD28 that mediate its role in the recovery of immunity against cancer in response to immune check-point blockade (ICB). Recently, we have uncovered a novel function for CD28 in the trafficking of T-cells to sites of infection (paper in preparation).
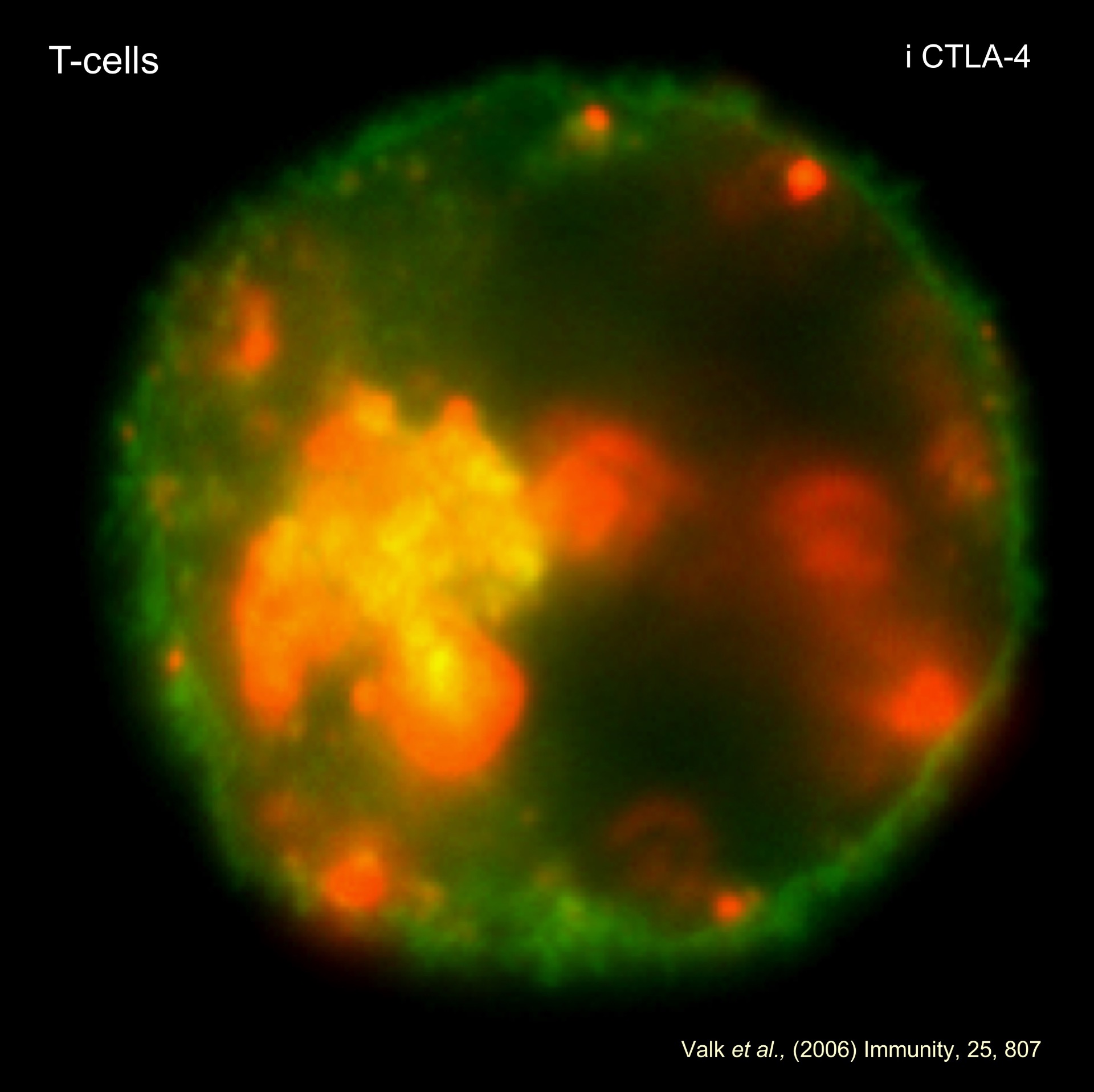
- GSK-3 and co-receptor blockade in immunotherapy. The past 15 years has witnessed a revolution in the application of immunotherapy for the treatment of various human cancers. Immune check-point blockade (ICB) uses monoclonal antibodies (MAbs) to block the binding of inhibitory receptors (IRs) to their natural ligands. CTLA-4 and PD-1 negatively regulate T-cell response. We have defined pathways that up-regulate CTLA-4 expression on T-cells and have reported a novel function for CTLA-4 where it up-regulates T-cell motility and over-riding the T cell receptor (TCR)-induced stop signal required for stable conjugate formation between T cells and antigen-presenting cells. Our model (reverse stop signal model) provides a new way of viewing how CTLA-4 modulates the threshold of signaling and autoimmunity. We are exploring how intervention in these functions can be applied to anti-tumor immunity. In one model, reverse stop signaling increases the efficacy of immunotherapy by limiting the contact times of CD8+ CTLs with tumors and thereby increasing the frequency of CTL-tumor interactions for more effective stochastic killing.
- Our laboratory has uncovered the central signaling pathway controls PD-1 expression in T-cells. We have showne that the serine/threonine kinase glycogen synthase kinase 3 (GSK-3) that controls PD-1 transcription and have developed a small molecule inhibitor (SMI) approach to down-regulate PD-1 expression. GSK-3 blockade is effective as anti-PD-1 in mouse models of cancer and can synergize with anti-PD-1 to inhibit the growth of ICB resistant solid tumors. Using CyTOF, projects have identified novel populations of tumor infiltrating T-cells (TILs) that arise due to combination therapy. We are presently developing a consortium to apply these findings to the clinic in the immune therapeutic treatment of patients with cancer.
- Defining Adaptor Function in T-cell immunity. Our laboratory identified novel adaptors: adhesion and degranulation-promoting adaptor protein (ADAP; encoded by FYB) and src kinase-associated phosphoprotein of 55 kDa (SKAP-55; encoded by SKAP1) that are responsible for controlling T-cell adhesion and motility. T-cell motility is an important feature of the immune system that is needed for the movement of T-cells to sites of inflammation/infection, lymph nodes and for interactions with antigen-presenting dendritic cells (DCs). We showed that the two adaptors are expressed primarily in T-cells where SKAP1 controls a pathway involving Rap1 and RapL and their binding to the cytoplasmic tail for LFA-1 activation. In additional studies, we have shown that different receptors such as LFA-1 can alter the composition of the LAT signalosome by displacing the GADs-SLP-76 module for T-cell motility. The identification of these signaling pathways represents a significant advance in defining the TCR 'inside-out' pathways needed for the 'stop-signal' that reduces motility for T-cell interactions with DCs. Projects involve assessing the composition of the pathways and to identify connections between mediators/interactions that regulate specific immune functions.
- Defining the function of immune-specific adaptors in T-cell function and cancer. We showed that Skap-1-/- deficient mice are resistant to the induction of collagen-induced arthritis (CIA), while a mutant of ADAP blocks the transmission of the human immunodeficiency virus (HIV-1) by impairing cell-to-cell transmission and viral spread. Our focus now is on immunotherapy and cancer where projects involve using retrovirus' encoding specific mutants of RapL, SKAP1 and SLP-76 to enhance the localization and amplitude of in vivo responses against different cancer types that include melanoma and lymphomas.
- Defining the function of the SLP-76-RanGAP1 regulation of the nuclear pore complex in T-cells. We uncovered a novel pathway involving the adaptor SLP-76 binding to RanGAP1 of the cytoplasmic filaments of the nuclear pore complex (NPC). The interaction controls >50% of T-cell receptor-induced transcription factor (NFAT-c1 and Rel-A) entry into the nucleus of T-cells. A single mutation in a single adaptor that disrupts NPC transport can inhibit T-cell responses by >80%. Projects involve uncovering the mechanisms by which SLP-76 activates RanGAP1, the nature of transcription factors affected by this pathway and its impact of the nature of the immune response to foreign antigen and cancer neoantigens.